Malaria research
October 27th, 2016
I am pleased to announce our latest research project: my staff and I are participating in the following very important initiative.
3 Day Malarone Acceptability and Tolerability research project (3MAT)
Malaria is one of the most common causes of fever in Australian travellers, with approximately 400 cases reported each year in Australia. Most travellers who develop malaria did not take anti-malarial medications, or did not take the medications properly (e.g. forgot to take tablets). Malaria is a serious illness and can potentially be fatal.
A research project is being conducted with the aim to make it easier and cheaper to take malaria pills and thus reduce the risk of malaria in travellers.
Malarone is a safe and effective anti-malarial medication. The standard dosage of Malarone for prevention of malaria is one tablet per day, starting 2 days before travelling to a malaria area, daily while in a malaria area, and continuing until 7 days after leaving the malaria area.
Research has shown that when people are given a 3-day course of Malarone for treatment of malaria, they are protected from malaria for at least 4 weeks afterwards.
It is therefore possible to protect travellers for 4 weeks by giving them just a 3-day course of Malarone before they go, and no tablets while they are away or on return. This research will assess the acceptability and compliance of the 3-day schedule of Malarone for malaria prevention.
Participants are currently being recruited from two specialist travel medicine clinics : OURS and the Travel Bug Vaccination Clinic in Adelaide
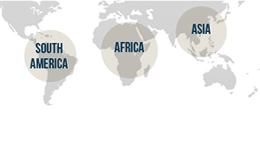
Australians 18 years of age and over, travelling to malaria endemic countries in Asia, the Pacific Islands, or South/Central America for 4 weeks or less are eligible to participate. The study aims to recruit approximately 220 travellers.
On completion of the study, the research team will publish the results in an internationally recognised medical journal so this important information can be shared with the medical and scientific community to improve the health of travellers. Individuals will not be identified when results are presented or published.
This project does not have any specific funding. It is being conducted by the doctors and nurses at the participating clinics at their own cost, with the aim of improving clinical best practice in travel medicine. Participants pay for their own medication.
To participate in the study, you will be given more information and be requested to attend a pre-travel consultation, take anti-malarial medications, fill in a symptom diary, and complete 3 questionnaires: the day of the visit, the day before departure and one week after return.
Participants will receive detailed information about the trial prior to participation, and can withdraw at any time.
For further information contact the participating clinics
(myself) Dr. Deborah Mills (Brisbane) Phone: 61 7 3221 90661
NP/RN. Lani Ramsey (Adelaide) Phone: 61 8 8267 3544
This project has ethics approval and is being conducted by the
Travel Medicine Alliance in conjunction with the Australian National University in Canberra.
References for 3mat Project
1. Behrens RH, Taylor RB, Pryce DI, Low AS. Chemoprophylaxis compliance in travelers with malaria. J Travel Med 1998;5(2):92-4.
2. Jute S, Toovey S. Knowledge, attitudes and practices of expatriates towards malaria chemoprophylaxis and personal protection measures on a mine in Mali. Travel Med Infect Dis 2007;5(1):40-3.
3. Laver SM, Wetzels J, Behrens RH. Knowledge of malaria, risk perception, and compliance with prophylaxis and personal and environmental preventive measures in travelers exiting Zimbabwe from Harare and Victoria Falls International airport. J Travel Med 2001;8(6):298-303.
4. Pistone T, Guibert P, Gay F, Malvy D, Ezzedine K, Receveur MC, et al. Malaria risk perception, knowledge and prophylaxis practices among travellers of African ethnicity living in Paris and visiting their country of origin in sub-Saharan Africa. Trans R Soc Trop Med Hyg 2007;101(10):990-5.
5. Piyaphanee W, Wattanagoon Y, Silachamroon U, Mansanguan C, Wichianprasat P, Walker E. Knowledge, attitudes, and practices among foreign backpackers toward malaria risk in southeast Asia. J Travel Med 2009;16(2):101-6.
6. Resseguier N, Machault V, Ollivier L, Orlandi-Pradines E, Texier G, Pradines B, et al. Determinants of compliance with malaria chemoprophylaxis among French soldiers during missions in inter-tropical Africa. Malaria J 2010;9:41.
7. Ropers G, Du Ry van Beest Holle M, Wichmann O, Kappelmayer L, Stuben U, Schonfeld C, et al. Determinants of malaria prophylaxis among German travelers to Kenya, Senegal, and Thailand. J Travel Med 2008;15(3):162-71.
8. Robinson P, Jenney AW, Tachado M, Yung A, Manitta J, Taylor K, et al. Imported malaria treated in Melbourne, Australia: epidemiology and clinical features in 246 patients. J Travel Med 2001;8(2):76-81.
9. Shanks GD, Ragama BO, Oloo AJ. Time to reappearance of malaria parasites following various drug treatment regimens in a holoendemic area of western Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(3):304-5.
10. Polhemus ME, Remich S, Ogutu B, Waitumbi J, Lievens M, Ballou WR, et al. Malaria treatment with atovaquone-proguanil in malaria-immune adults: implications for malaria intervention trials and for pre-exposure prophylaxis of malaria. Antimicrobial agents and chemotherapy 2008;52(4):1493-5.
11. Edstein MD, Kotecka BM, Anderson KL, Pombo DJ, Kyle DE, Rieckmann KH, et al. Lengthy antimalarial activity of atovaquone in human plasma following atovaquone-proguanil administration. Antimicrobial agents and chemotherapy 2005;49(10):4421-2.
12. Butcher GA, Sinden RE. Persistence of atovaquone in human sera following treatment: inhibition of Plasmodium falciparum development in vivo and in vitro. The American journal of tropical medicine and hygiene 2003;68(1):111-4.